Category: Medications - Page 3
Learn what lab tests you actually need while on statins - and which ones are unnecessary. Stop worrying about liver enzymes and focus on what really matters for your heart health.
View DetailsGeneric drugs cost far less than brand-name versions, but the labor behind them is more complex than it seems. Learn how production scale, global outsourcing, and quality control shape labor costs in pharmaceutical manufacturing.
View DetailsPharmacy warning icons on medication labels are designed to prevent dangerous mistakes, but many people misunderstand them. Learn what the most common symbols mean, why inconsistencies cause errors, and how to stay safe.
View DetailsLearn how to time your medications correctly to avoid dangerous interactions and side effects. Simple changes in when you take pills can boost effectiveness and prevent hospital visits.
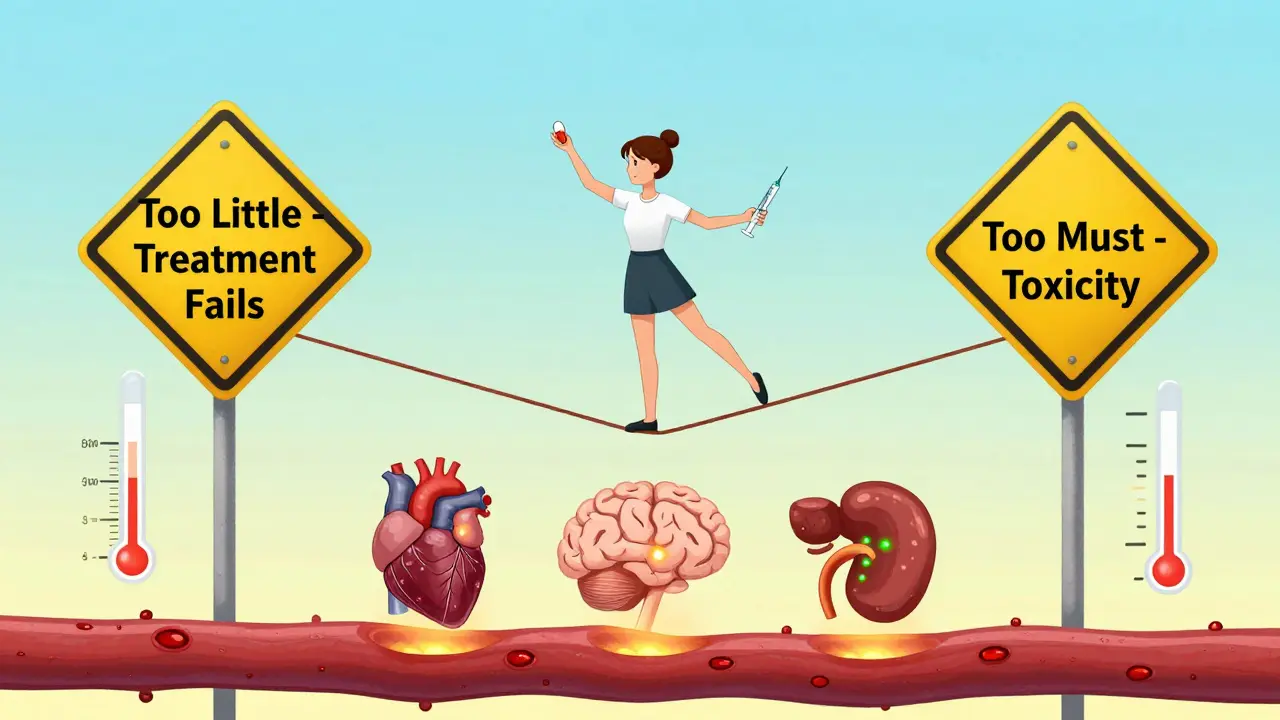
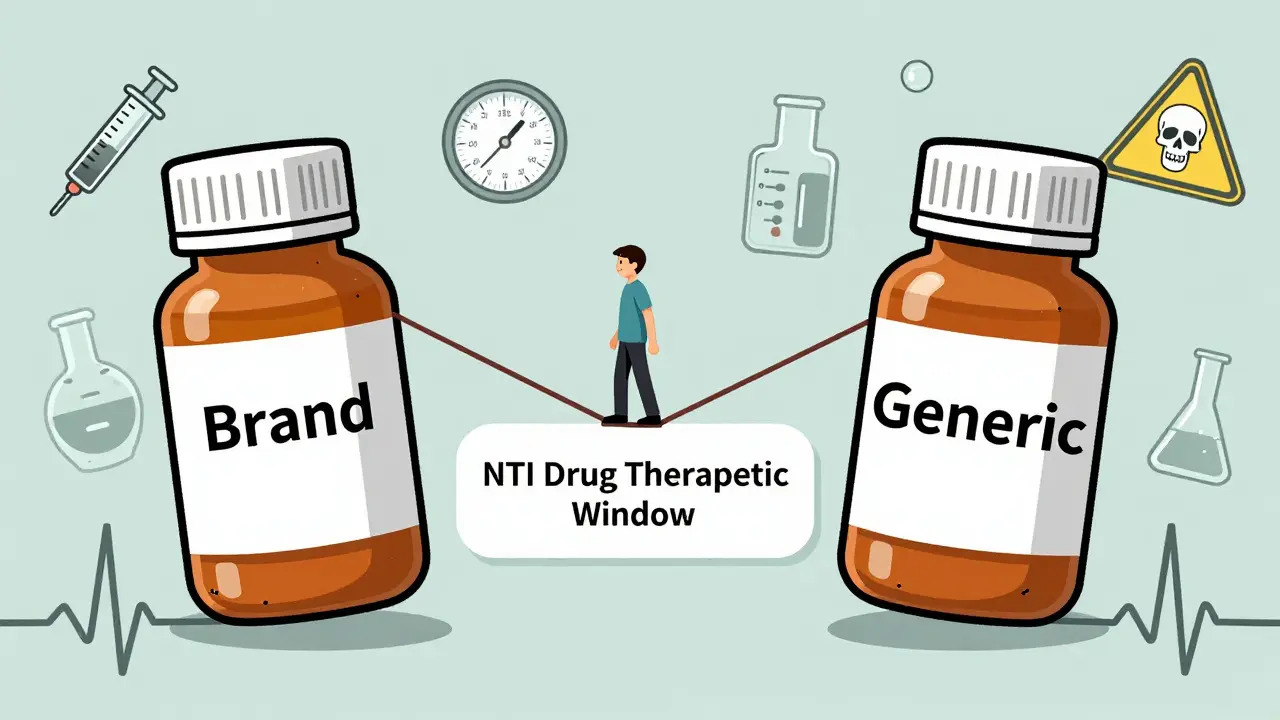
View DetailsNTI drugs have a tiny margin between effective and toxic doses. Learn which common medications like warfarin, lithium, digoxin, and levothyroxine fall into this high-risk category, why switching generics can be dangerous, and how to stay safe with proper monitoring.
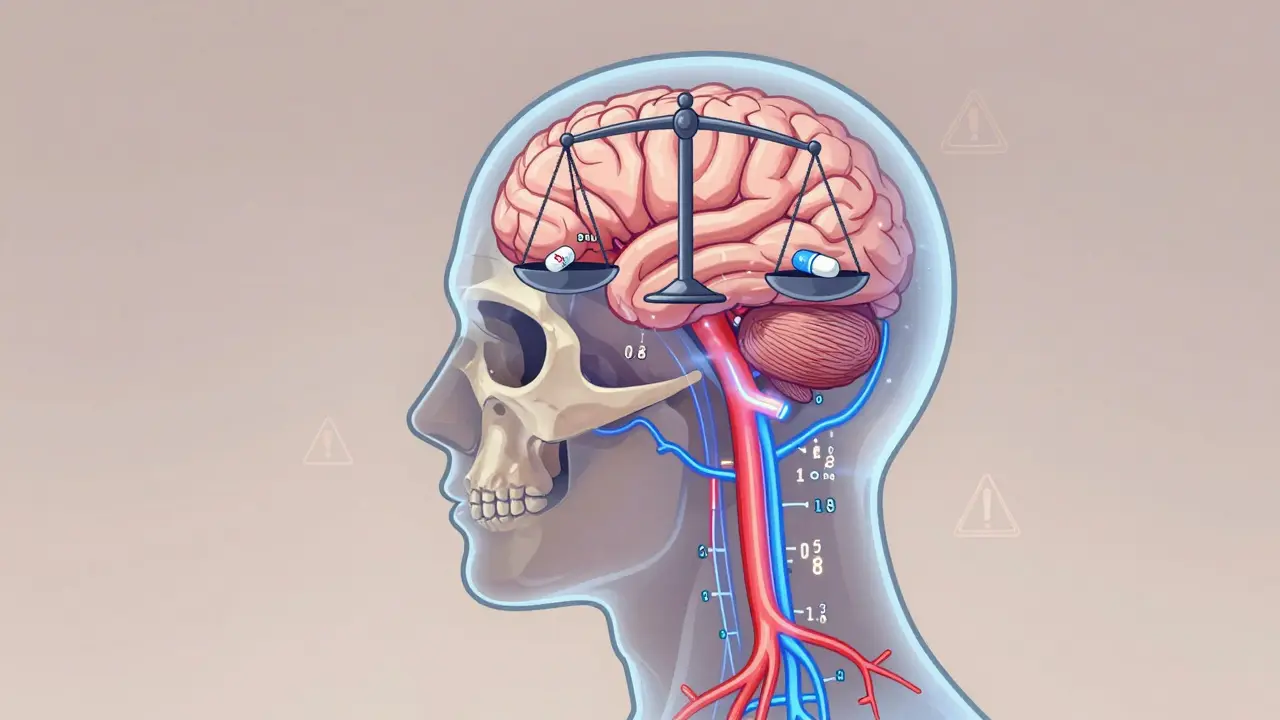
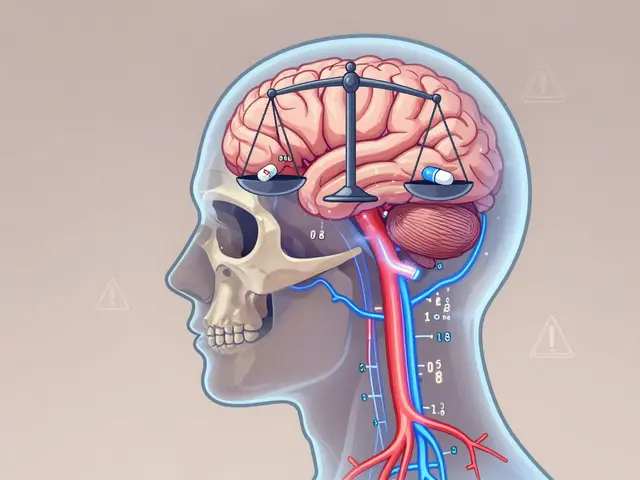
View DetailsLithium carbonate generics require careful serum level monitoring due to narrow therapeutic ranges and formulation differences. Even bioequivalent generics can affect blood levels differently, risking toxicity or reduced efficacy in bipolar disorder treatment.
View DetailsGeneric and brand-name drugs contain the same active ingredient, so their risk for drug interactions is identical. Regulatory standards ensure bioequivalence, and real-world data confirms no higher risk with generics.
View DetailsDecision aids help patients understand the real risks and benefits of switching medications, especially when side effects are a concern. They use clear visuals, personal values, and evidence to guide shared decisions with providers.
View DetailsStudies show that switching to generic versions of narrow therapeutic index (NTI) drugs like warfarin, phenytoin, and cyclosporine can lead to dangerous fluctuations in blood levels. While some patients remain stable, others face seizures, organ rejection, or bleeding. Monitoring is critical after any switch.
View DetailsInsurers use preferred generic lists to cut drug costs and lower premiums. These formularies steer patients toward cheaper, equally effective generics - saving billions annually. Learn how they work, where they fall short, and how to navigate them.
View Details