When you pick up a prescription, you might see two options: the familiar brand-name pill or a cheaper generic version. Many people wonder - does switching to a generic change how the drug interacts with other medicines you’re taking? The short answer: no. The risk of drug interactions is essentially the same for generic and brand-name medications.
Why the confusion exists
It’s easy to assume that because generics cost less, they must be different. But the difference isn’t in the active ingredient. Both brand-name and generic drugs contain the exact same chemical compound that treats your condition. If your brand-name medication is lisinopril for high blood pressure, the generic version is also lisinopril - same molecule, same effect. The confusion comes from two things: inactive ingredients and personal experiences. Generics can use different fillers, dyes, or preservatives. For most people, that doesn’t matter. But if you’re allergic to lactose, and one version of your generic pill uses lactose as a binder while the brand doesn’t, you might notice stomach upset. That’s not a drug interaction - it’s a reaction to an additive. And sometimes, when people switch to a cheaper version and feel different, they blame the generic. But studies show this is often the nocebo effect - the opposite of placebo. You expect something to go wrong, so you notice normal side effects more.How regulators ensure safety
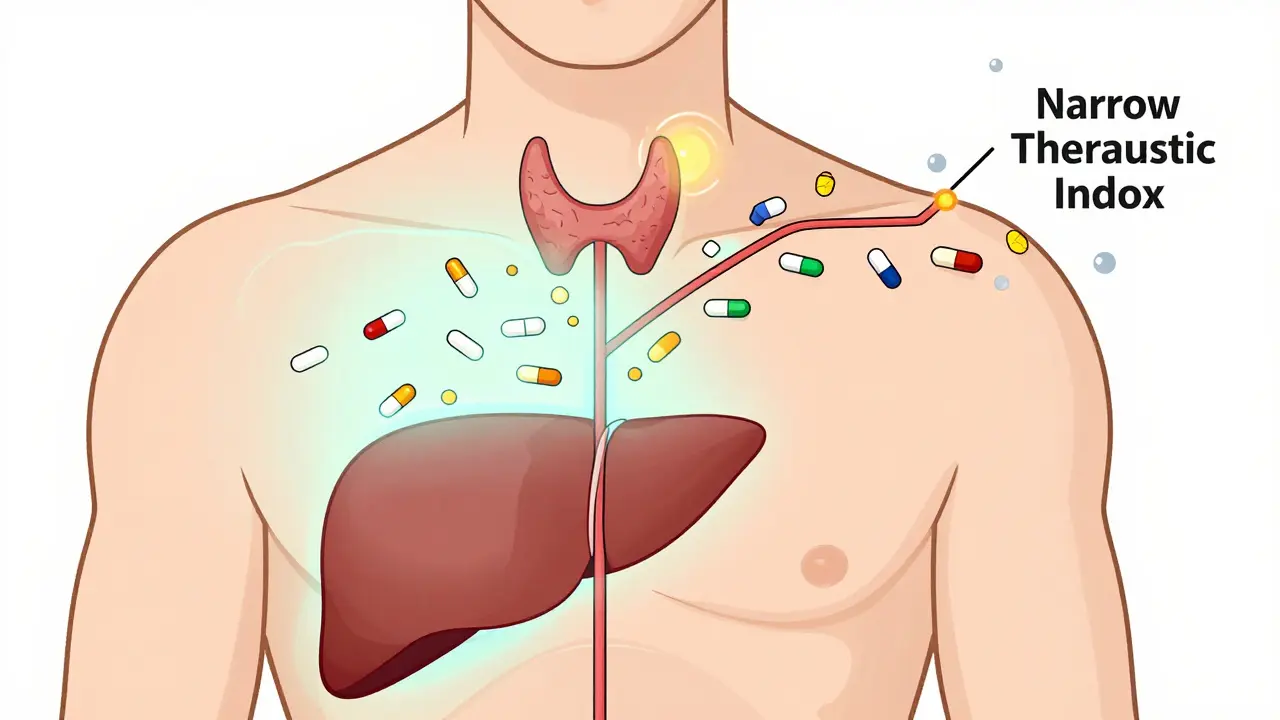
The U.S. Food and Drug Administration (FDA) doesn’t just approve generics because they look similar. They require strict proof of bioequivalence. That means the generic must deliver the same amount of active drug into your bloodstream at the same rate as the brand. The acceptable range? Between 80% and 125% of the brand’s absorption levels. That’s not a wide gap - it’s a tight band designed to make sure your body responds the same way. For most drugs, this works perfectly. But for drugs with a narrow therapeutic index - like warfarin, lithium, or levothyroxine - even small changes in blood levels can matter. That’s why the FDA requires tighter standards for these: a 90% to 111% bioequivalence range. In these cases, pharmacists are trained to check if you’ve switched between different generic brands. A change in formulation, even if both are generic, could theoretically affect how the drug interacts with others.Real-world data doesn’t support higher risk
A 2020 study in Scientific Reports followed over 100,000 patients on 17 different cardiovascular drugs. The results? People taking generic versions had fewer heart attacks, strokes, and deaths than those on brand-name drugs. The study adjusted for age, income, and other health conditions. The difference wasn’t because generics were stronger - it was because more people stuck with their treatment when it was affordable. Drug interaction reports tell the same story. Between 2015 and 2020, the FDA’s adverse event database recorded 0.78% of brand-name drug users reporting interactions. For generics, it was 0.82%. That tiny difference? Statistically meaningless. No higher risk. No pattern. Just noise.
What actually causes drug interactions
Drug interactions happen because of the active ingredient - not whether it’s branded or generic. If you’re on blood thinners and start taking St. John’s wort, you’re at risk whether the blood thinner is Coumadin or warfarin. If you take an antibiotic and then drink grapefruit juice, the interaction comes from the antibiotic’s chemical structure - not its label. The real culprits are:- Multiple medications taken together
- Herbal supplements like ginseng or garlic extract
- Alcohol or caffeine
- Food that blocks absorption (like dairy with some antibiotics)
What patients should do
If you’re worried about interactions after switching to a generic, here’s what actually helps:- Keep a list of all your medications - including supplements and over-the-counter drugs.
- Ask your pharmacist to check for interactions every time you pick up a new prescription.
- If you notice new side effects after switching, don’t assume it’s the generic. Write down what changed: timing, dosage, other meds, diet.
- For critical drugs like thyroid medication or seizure meds, ask your doctor to write “dispense as written” on the prescription if you’ve had stability on one brand or generic.



Olivia Goolsby
26 December / 2025Okay, but have you ever actually looked at the FDA’s approval process for generics? It’s a joke. They approve them based on bioequivalence in healthy adults, but what about elderly patients with kidney disease? Or people on five different meds? The system is rigged. Big Pharma pays off regulators, and then they slap a generic label on it and charge half the price-while still making billions. I switched to a generic for my thyroid med, and within two weeks I was sweating through my sheets at 3 a.m. Coincidence? I think not. They don’t test for long-term interaction risks because they don’t want to find out the truth. The FDA’s 80–125% range? That’s a loophole big enough to drive a truck through. And don’t get me started on the fillers-talc, corn starch, lactose-some of these generics are basically pharmaceutical junk food.