Tag: NTI drugs
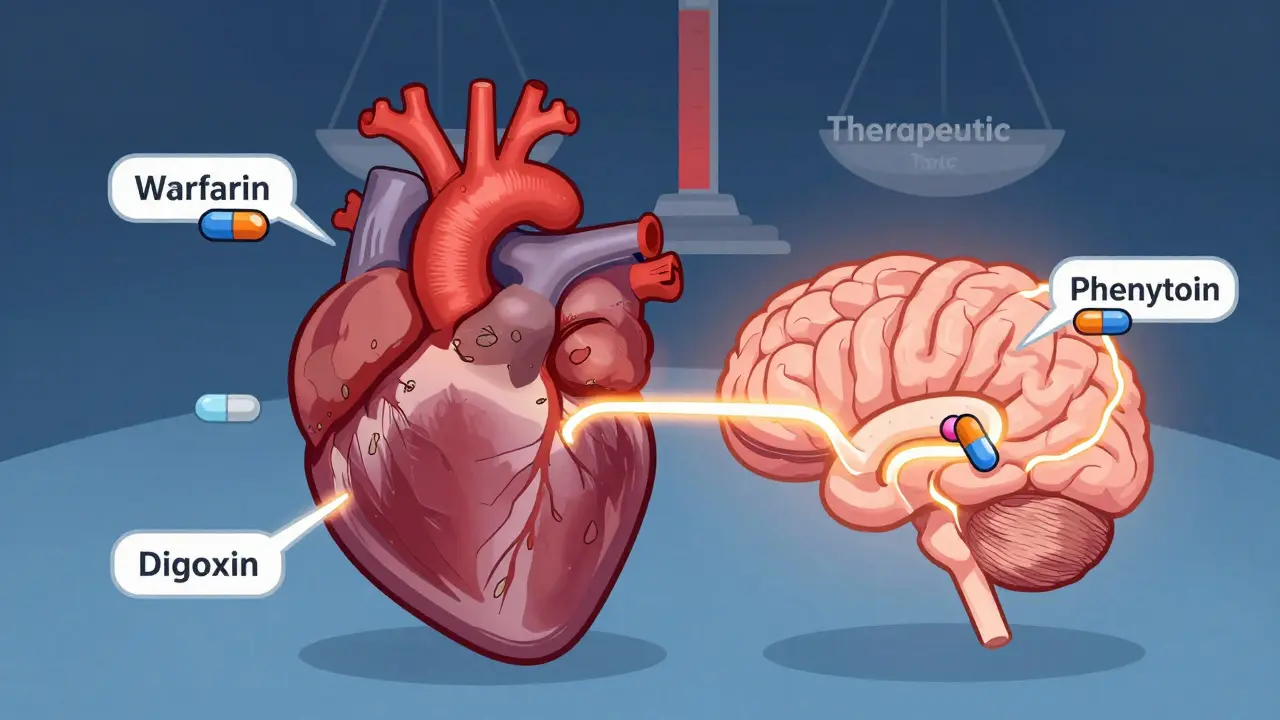
The FDA enforces stricter bioequivalence rules for NTI drugs like warfarin, digoxin, and phenytoin, requiring tighter potency limits and variability controls to ensure patient safety. Learn how these standards differ from regular generics.
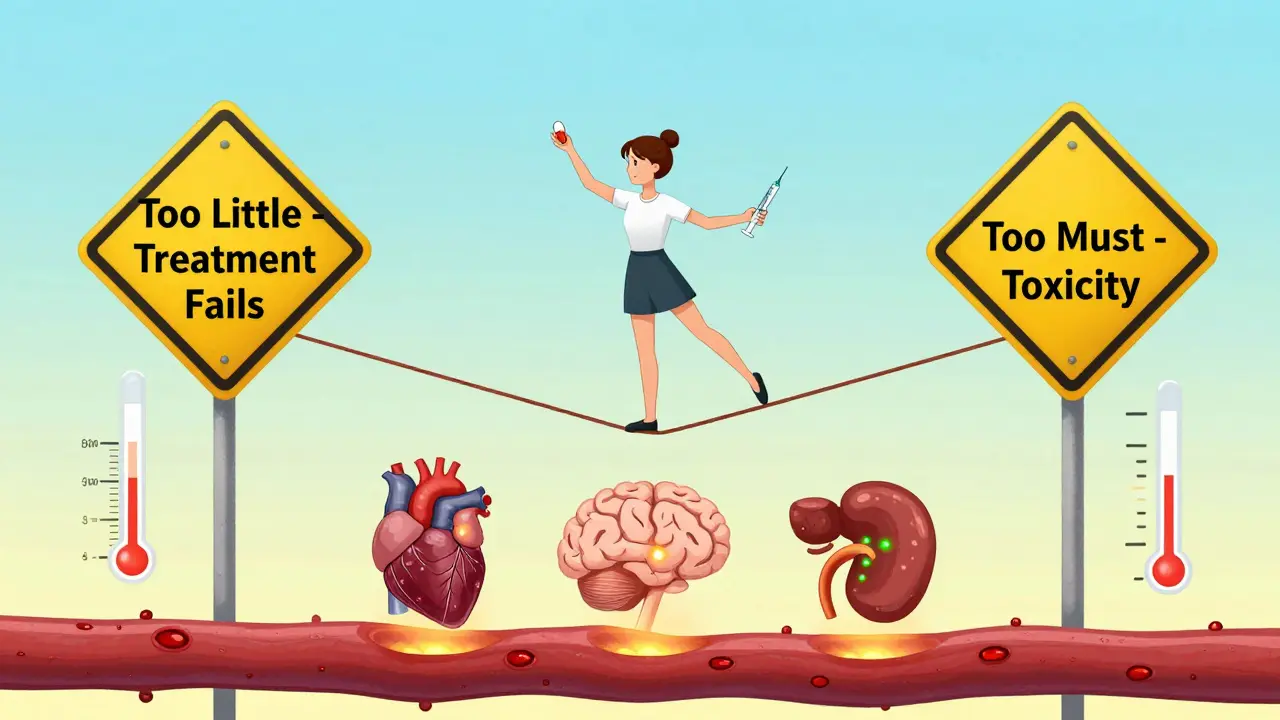
View DetailsNTI drugs have a tiny margin between effective and toxic doses. Learn which common medications like warfarin, lithium, digoxin, and levothyroxine fall into this high-risk category, why switching generics can be dangerous, and how to stay safe with proper monitoring.
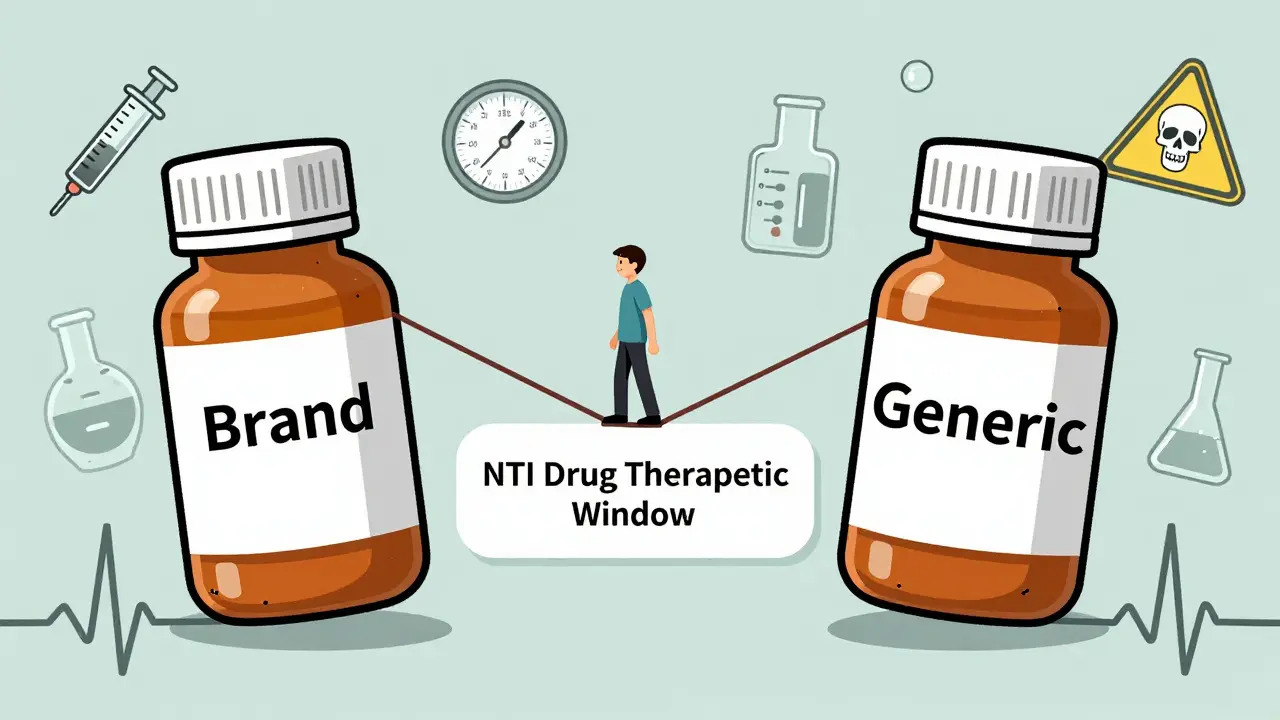
View DetailsStudies show that switching to generic versions of narrow therapeutic index (NTI) drugs like warfarin, phenytoin, and cyclosporine can lead to dangerous fluctuations in blood levels. While some patients remain stable, others face seizures, organ rejection, or bleeding. Monitoring is critical after any switch.
View Details