Generic Drugs: What They Are, How They Save Money, and What You Need to Know
When you hear generic drugs, pharmaceutical products that contain the same active ingredients as brand-name drugs but are sold without a brand name. Also known as generic medications, they are legally required to work the same way, in the same amount, and with the same safety profile as their brand-name counterparts. Yet many people still wonder: are they really the same? The answer is yes—if the FDA has approved them. And that approval isn’t just a stamp; it’s a rigorous process that ensures the generic version matches the original in strength, dosage, and how your body absorbs it.
What makes generic drug manufacturers, companies that produce FDA-approved versions of brand-name drugs after patents expire so powerful is competition. When just one company makes a generic, prices might drop 20-30%. But when four or more manufacturers enter the market, prices can plunge over 70%. That’s not theory—it’s real data from the FDA and independent studies. This is why drugs like metformin or amoxicillin cost pennies today, while the same pills were once hundreds of dollars. The system works when there are enough players. But when one company holds a patent too long, or when litigation delays entry, prices stay high and patients pay the price.
Not all generics are created equal, though. FDA approval, the official process that verifies a generic drug meets strict standards for safety, effectiveness, and quality is the only thing standing between a safe product and a risky one. Tentative approval means a company is ready to launch but is waiting for a patent to expire. That’s why you might see the same drug available at one pharmacy but not another—it’s not about quality, it’s about timing and legal battles. And while biosimilars are often grouped with generics, they’re different: they’re complex biologic drugs copied from other biologics, not simple chemical pills. They’re cheaper than the original biologics, but not as cheap as traditional generics.
You’ll find posts here that explain why some people get rashes on lamotrigine, how to take pills with food for better absorption, and how to avoid dangerous interactions with MAO inhibitors. You’ll learn how to report side effects to the FDA, how to handle getting the wrong medication from the pharmacy, and why carrying pills in original containers matters when you travel. There’s even a piece on how timolol eye drops pollute waterways—because even cheap generics have environmental costs.
What ties all these together? Real people using real drugs. Whether you’re on metformin and worried about B12 loss, switching antidepressants, or just trying to save money on your statin, the information here isn’t theoretical. It’s practical. It’s tested. It’s what you need to know before you take the next pill.
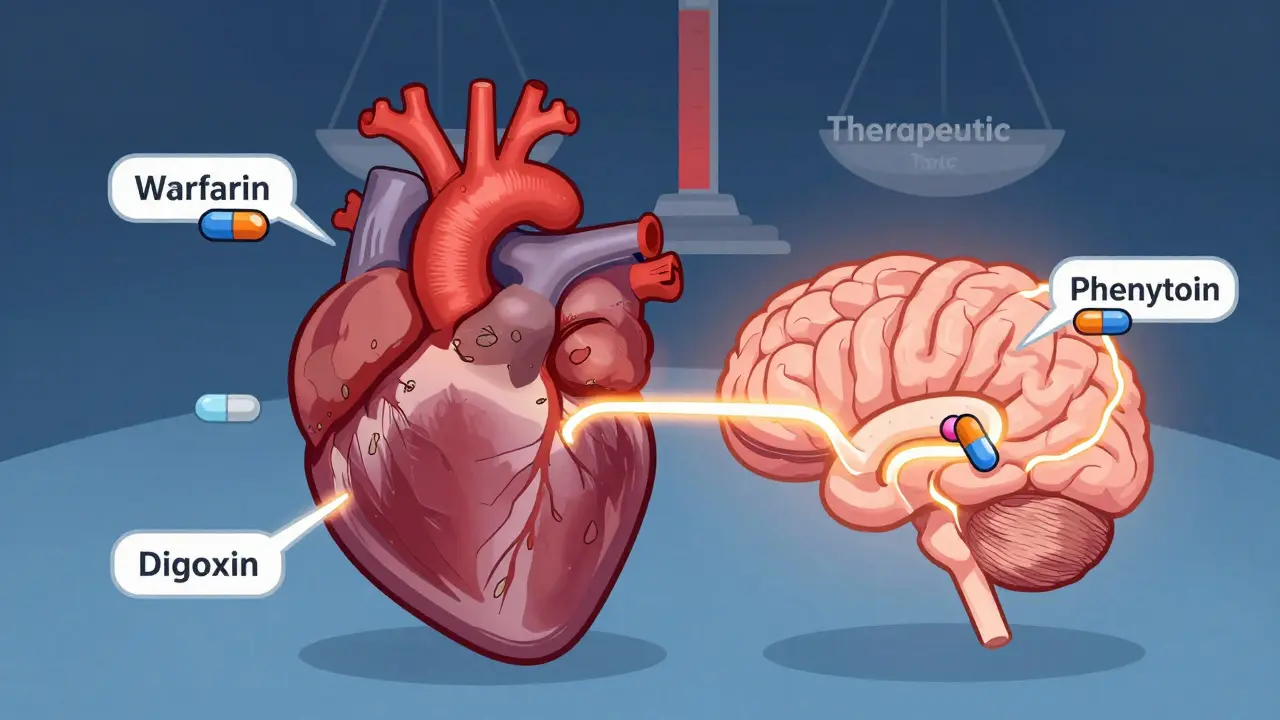
The FDA enforces stricter bioequivalence rules for NTI drugs like warfarin, digoxin, and phenytoin, requiring tighter potency limits and variability controls to ensure patient safety. Learn how these standards differ from regular generics.
View DetailsGeneric and brand-name drugs contain the same active ingredient, so their risk for drug interactions is identical. Regulatory standards ensure bioequivalence, and real-world data confirms no higher risk with generics.
View DetailsInsurers use preferred generic lists to cut drug costs and lower premiums. These formularies steer patients toward cheaper, equally effective generics - saving billions annually. Learn how they work, where they fall short, and how to navigate them.
View DetailsTrack how your body reacts when switching to generic medications with a simple medication journal. Learn what to record, why it matters, and how to use it to protect your health.
View Details