Market Entry Delay: Why Generic Drugs Take Longer to Reach Patients
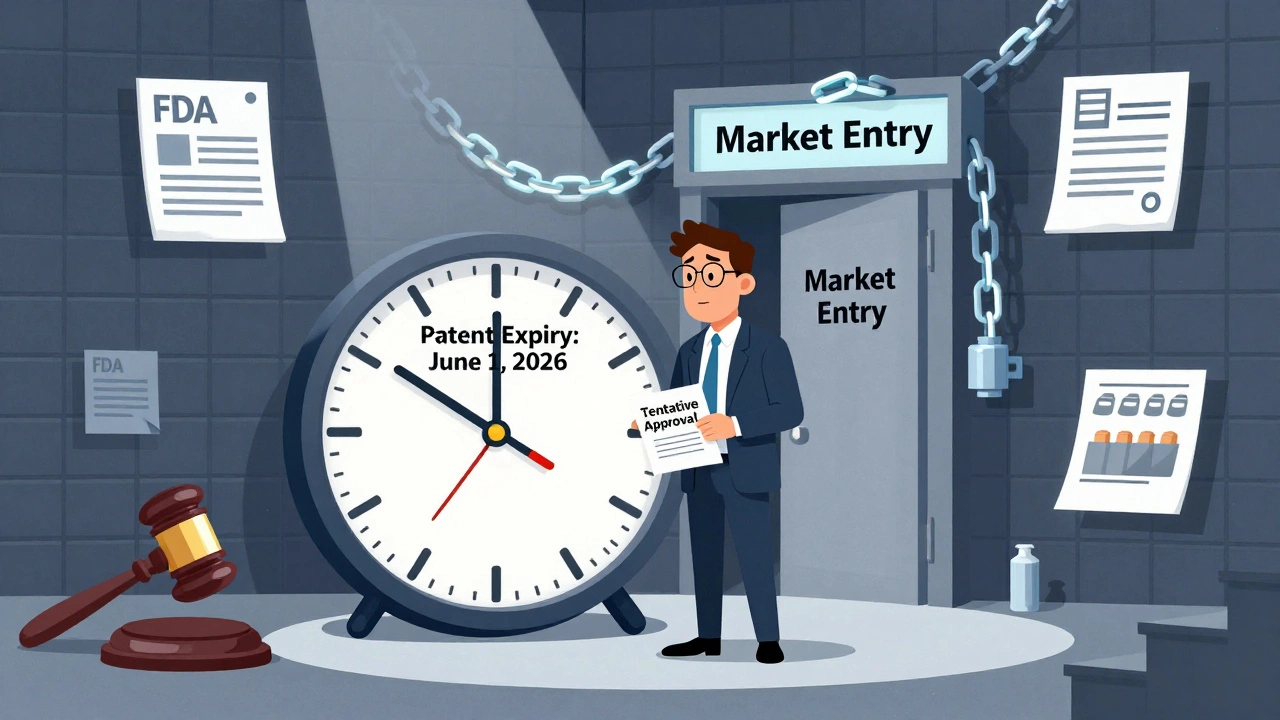
When a brand-name drug loses its patent, a market entry delay, the time between a patent expiration and the first generic version hitting shelves. Also known as generic drug lag, it’s when patients are stuck paying high prices even though cheaper alternatives exist. This isn’t just a paperwork issue—it’s a financial burden on millions. In the U.S., some generics take over a year to appear after patent expiry, even when manufacturers are ready to produce them.
Why does this happen? The FDA approval, the government process that clears generic drugs for sale. Also known as ANDA review, it’s meant to ensure safety—but bottlenecks, staffing shortages, and complex submissions slow it down. One manufacturer might file early, but if another has a patent challenge or a manufacturing hiccup, the whole line gets held up. And when only one or two companies make a drug, prices don’t drop fast. But when generic manufacturer, a company that produces non-branded versions of brand-name drugs. Also known as generic pharma, it enters the market—especially if there are four or more—the price can drop over 70%. That’s the power of competition. But too often, the system doesn’t let enough players in.
Some delays come from legal battles—brand companies file lawsuits to block generics, using tactics like "patent thickets" or minor reformulations to extend exclusivity. Others come from supply chain issues: a single factory failing an inspection can delay an entire drug line. And while the FDA has tried to speed things up with fast-track programs, backlogs still pile up. The result? Patients wait longer for affordable meds, pharmacies can’t stock cheaper options, and insurers pay more than they should.
What’s worse, this delay hits hardest for drugs with narrow therapeutic indexes—where even small differences in dosage matter. Think epilepsy meds like lamotrigine or blood thinners. If a generic isn’t available, patients might get stuck with a brand version they can’t afford, or worse, skip doses. And when they finally get the generic, they might not know how to track their response—something a medication journal can help with.
But it’s not all bad news. When multiple generic manufacturer entries happen, prices fall fast. And new business models—like direct-to-consumer pharmacies—are cutting out middlemen to get these drugs to people faster. Still, the system needs more transparency, faster reviews, and fewer legal roadblocks.
Below, you’ll find real guides on how generic drugs work, how to save money when they finally arrive, what to watch for when switching, and how to report problems when they don’t. This isn’t theory—it’s what’s happening right now in pharmacies, clinics, and patients’ medicine cabinets.
Tentative approval from the FDA lets generic drug makers prepare for market entry before patents expire, but litigation and timing mistakes can cause costly delays. Learn how the Hatch-Waxman Act shapes the race to bring affordable drugs to patients.
View Details