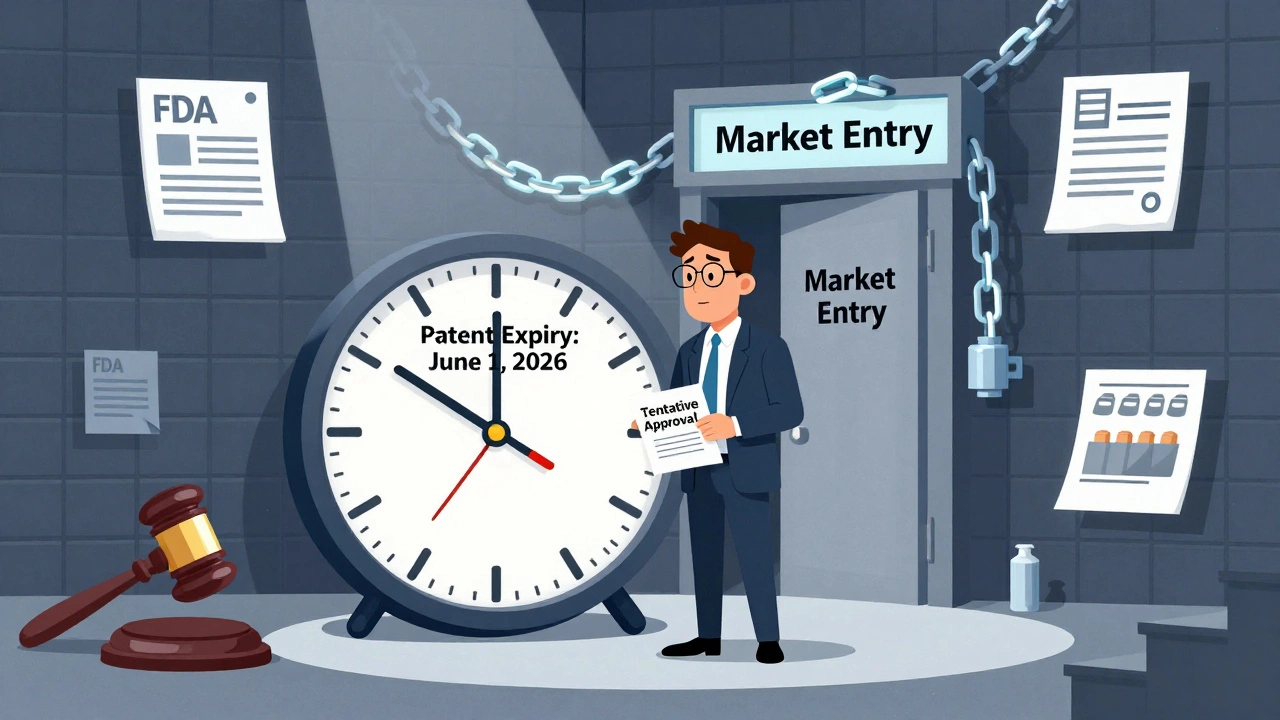
When a generic drug company gets a letter from the FDA saying their application is tentatively approved, it’s not a celebration-it’s the start of a long, high-stakes wait. They’ve passed every scientific test. Their pills match the brand-name drug in strength, safety, and quality. But they can’t sell them. Not yet. Why? Because a patent is still active. And that patent is being fought in court.
This isn’t a glitch in the system. It’s the whole point.
The U.S. Food and Drug Administration (FDA) created the tentative approval process under the Hatch-Waxman Act of 1984. That law was designed to balance two things: protecting innovation by giving brand-name drug makers exclusive rights, and getting cheaper generic versions to patients as soon as those rights expire. Tentative approval is the middle ground. It lets the FDA finish reviewing the generic application early, so when the patent finally falls, the drug can hit the market in weeks-not years.
But here’s what most people don’t realize: tentative approval doesn’t mean the race is over. It means the real game has just begun.
What Tentative Approval Really Means
Tentative approval is not approval. It’s a holding pattern. The FDA has reviewed every batch of data-stability tests, bioequivalence studies, manufacturing processes-and found nothing wrong. The application is scientifically complete. But the agency can’t issue final approval because a patent or exclusivity period is still in effect.
For example, if a brand-name drug has a patent that expires on June 1, 2026, and a generic company submits an ANDA with a Paragraph IV certification challenging that patent, the FDA will issue a tentative approval as soon as the scientific review is done-even if that happens in early 2025. The company now has a seat at the table. They’re next in line. But they can’t open the door until the patent expires.
According to FDA data from 2023, about 1,000 ANDAs receive tentative approval each year. That’s roughly 85% of all generic drugs entering the market that face patent barriers. It’s the standard path for anyone serious about competing in the U.S. generic market.
The key difference between tentative and final approval? One lets you sell. The other just lets you wait.
The Litigation Trap
Tentative approval is almost always tied to patent litigation. That’s because generic companies don’t just wait-they fight. When they file an ANDA, they can submit a Paragraph IV certification, which says, "This patent is invalid, or we don’t infringe it."
That triggers a legal bomb. The brand-name company has 45 days to sue. If they do, the FDA is legally required to delay final approval for up to 30 months. That’s called a "30-month stay." It’s not a guarantee the patent will hold up. It’s just a pause button.
During this time, the generic company sits with tentative approval. They can’t sell. But they can prepare. They can line up distribution, train sales reps, print labels. They can even start building relationships with pharmacies. All while the courts decide whether the patent is real or just a tactic to delay competition.
Some companies win. In 2018, Lupin Limited got tentative approval for its generic version of Cialis. When the patent expired, they submitted their final approval request. Within 24 hours, the FDA approved it. They captured 42% of the market in the first month.
Others lose. Aurobindo Pharma got tentative approval for a generic version of Jardiance in 2020. But they made a small change to their manufacturing site and didn’t document it properly. When the patent expired, their final approval request was rejected. The delay? Four months. Revenue loss? Around $150 million.
The Hidden Work of Waiting
Many assume that once you get tentative approval, you just sit back and wait for the patent to expire. That’s a dangerous myth.
The FDA requires companies to submit amendments-updates to their application-at least three months before the earliest possible approval date. If the change is minor, like updating a label, you need to file 90 days in advance. If it’s major-like switching suppliers or changing the formulation-you need 10 months.
Miss the deadline? Your approval gets pushed back. Even if the patent expires on January 1, you won’t get final approval until March if you filed late. That’s three months of lost sales.
And it’s not just about timing. The FDA also checks if your manufacturing site still meets current good manufacturing practices (cGMP). If there’s a compliance issue-say, a recent inspection flagged a problem-you’ll be stuck until you fix it. In 2022, 27% of delayed final approvals were due to cGMP issues, not patents.
Companies that succeed treat tentative approval like a live project. They track patent expiration dates down to the day. They have legal teams and regulatory teams talking every week. They don’t wait for the FDA to remind them. They file amendments early, even if they’re not sure the patent will be invalidated.

Why This System Exists
The Hatch-Waxman Act didn’t create this system to make life hard for generic companies. It was meant to encourage competition without killing innovation.
Brand-name drug makers spend billions developing new medicines. The law gives them up to 12 years of exclusivity (including patent and data protection) to recoup those costs. But once that time is up, the public deserves access to affordable alternatives.
Tentative approval is the bridge. It lets generic companies invest in production before the patent expires. It lets pharmacies plan inventory. It lets patients know a cheaper option is coming.
Without it, the market would be chaotic. Imagine every generic drug waiting until the patent expires to even start the FDA review. That could mean 18 months or more of delay. Patients would pay more. Hospitals would scramble. The whole system would slow down.
That’s why 92% of the top 50 generic manufacturers actively pursue tentative approval. It’s not optional-it’s essential.
The 180-Day Exclusivity Trap
There’s a big prize for the first company to challenge a patent successfully: 180 days of market exclusivity. During that time, no other generic can enter. That’s a huge financial advantage.
But here’s the catch: you have to actually launch. If you get tentative approval, win the lawsuit, and then sit on your approval for six months, you lose your exclusivity. The FDA will let others in.
That’s why some companies settle. They get paid by the brand-name company to delay launch. It’s called a "pay-for-delay" agreement. It’s legal, but controversial. The FTC has challenged dozens of these deals, arguing they keep prices high.
One 2023 study found that 15% of tentatively approved ANDAs never launch because the company either settled too early or misjudged the legal timeline. They got the approval. They had the right. But they didn’t move fast enough.

What Goes Wrong
The biggest mistakes? Timing and communication.
- Missing the 90-day amendment window for minor changes
- Failing to track pediatric exclusivity extensions (which can add 6 months)
- Not documenting manufacturing changes properly
- Assuming the patent expires on the date listed online (it doesn’t-legal filings can extend it)
- Not having a legal team review the patent landscape before filing
One company got tentative approval for a generic version of EpiPen. They thought the patent expired in June. It didn’t. There was a hidden pediatric exclusivity that added six months. They launched in June. The FDA blocked them. They lost six months of revenue and their 180-day exclusivity window.
Another company changed their packaging design without telling the FDA. They thought it was minor. The FDA said it was major. Their approval was delayed by four months.
These aren’t rare. Evaluate Pharma found that 15% of tentative approvals face delays in final approval due to procedural errors.
How to Get It Right
If you’re in the generic drug business, here’s how to avoid the traps:
- Track every patent and exclusivity period like a clock. Use a system, not a spreadsheet.
- File amendments at least 100 days before the earliest possible approval date-not 90.
- Have your legal and regulatory teams meet weekly during the tentative approval period.
- Don’t assume patent expiration dates from public databases. Get the official filing from the USPTO.
- Keep your manufacturing site in perfect compliance. One inspection failure can wipe out months of progress.
- Know your 180-day exclusivity clock. If you win the lawsuit, launch fast.
Companies that do this right don’t just survive-they dominate. Teva Pharmaceuticals used this system to launch a generic version of Januvia. They filed their final approval request exactly 90 days before patent expiry. No delays. No surprises. They captured 68% of the market in the first month.
What’s Next
The FDA is trying to speed things up. Starting in 2023, they promised to review final approval requests for tentatively approved ANDAs in 30 days instead of 60-90. That’s progress.
But the complexity is growing. New drugs-especially biologics and complex generics-are coming with layered patent portfolios. Some have 20+ patents. Some have patents on manufacturing methods, not just the active ingredient.
Analysts predict tentative approvals will rise 12% a year through 2027. The system isn’t going away. It’s getting more important.
For patients, that means more generics, faster. For companies, it means more pressure to get it right.
The system works-but only if you treat tentative approval like a live mission, not a waiting room.

sean whitfield
4 December / 2025So the FDA just lets Big Pharma stretch patents with legal smoke and mirrors? Classic. We're paying for medicine like it's a luxury car. And they call this innovation.