Tag: bioequivalence
26 Dec 2025
Generic and brand-name drugs contain the same active ingredient, so their risk for drug interactions is identical. Regulatory standards ensure bioequivalence, and real-world data confirms no higher risk with generics.
View Details
23 Dec 2025
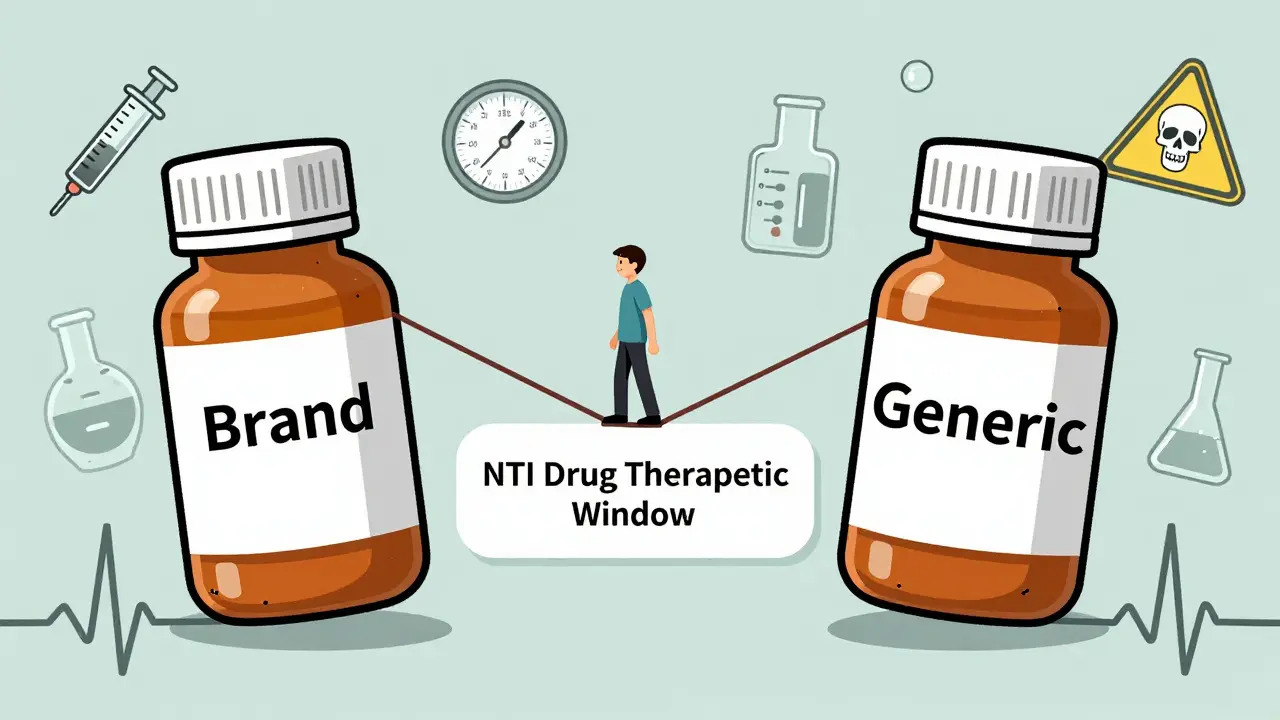
Studies show that switching to generic versions of narrow therapeutic index (NTI) drugs like warfarin, phenytoin, and cyclosporine can lead to dangerous fluctuations in blood levels. While some patients remain stable, others face seizures, organ rejection, or bleeding. Monitoring is critical after any switch.
View Details