When you hear the word biosimilar, you might think it’s just another name for a generic drug. But that’s not true. Biosimilars aren’t copies in the way aspirin or metformin are copies. They’re complex biological medicines made from living cells - and monoclonal antibody biosimilars are among the most advanced and impactful of them all.
What Makes Monoclonal Antibody Biosimilars Different?
Monoclonal antibodies are large proteins, each weighing about 150,000 daltons. That’s over 25 times heavier than insulin and nearly seven times heavier than growth hormone. Because they’re made by living cells - not synthesized in a lab like small-molecule drugs - no two batches are exactly alike. Even tiny differences in how cells fold the protein or attach sugar molecules (called glycosylation) can change how the drug works in the body. That’s why regulators like the FDA and EMA don’t require biosimilars to be identical to the original. Instead, they demand high similarity - no clinically meaningful differences in safety, purity, or effectiveness. To prove this, manufacturers run hundreds of lab tests, animal studies, and clinical trials. The goal isn’t to match every atom. It’s to show the body responds the same way.Approved Monoclonal Antibody Biosimilars and What They Treat
As of 2023, the FDA has approved over 20 monoclonal antibody biosimilars in the U.S. Here are the most common ones and the conditions they’re used for:- Bevacizumab biosimilars (like Mvasi, Zirabev, Vegzelma): Used for colorectal, lung, ovarian, and brain cancers. Six approved versions are now available, cutting treatment costs by up to 30%.
- Rituximab biosimilars (Truxima, Ruxience, Riabni): Treat non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, and rheumatoid arthritis. A 2022 JAMA Oncology study of 1,247 patients showed no drop in effectiveness when switching from Rituxan to Truxima - but a 28% drop in cost per cycle.
- Trastuzumab biosimilars (Ogivri, Herzuma, Kanjinti, Hercessi): Target HER2-positive breast and stomach cancers. Six FDA-approved versions have expanded access for patients who couldn’t afford the original Herceptin.
- Infliximab biosimilars (Remsima, Inflectra): Used for Crohn’s disease, ulcerative colitis, and rheumatoid arthritis. Remsima became the first monoclonal antibody biosimilar in the U.S. to be labeled interchangeable in July 2023, meaning pharmacists can swap it for the brand without a doctor’s new prescription.
- Adalimumab biosimilars (Hyrimoz, Amjevita, Cyltezo): These are replacing Humira, the world’s top-selling drug for years. Fourteen biosimilar candidates are in development, with Hyrimoz approved in late 2023.
These aren’t just cheaper versions. They’re bringing life-saving treatments to people who previously couldn’t get them - especially in countries without universal healthcare.
Why Cost Savings Matter
The original monoclonal antibody drugs often cost $10,000 to $20,000 per year. Biosimilars typically launch at 15% to 35% lower prices. In the U.S., the savings are expected to hit $250 billion between 2023 and 2028. Bevacizumab, trastuzumab, and rituximab biosimilars will account for 78% of that total. In one cancer center, switching to Truxima saved $1.2 million in a single year. That’s enough to fund 40 additional patient treatments. Hospitals and insurers are pushing hard for biosimilars - not because they’re cheaper to make, but because they’re cheaper for patients to use.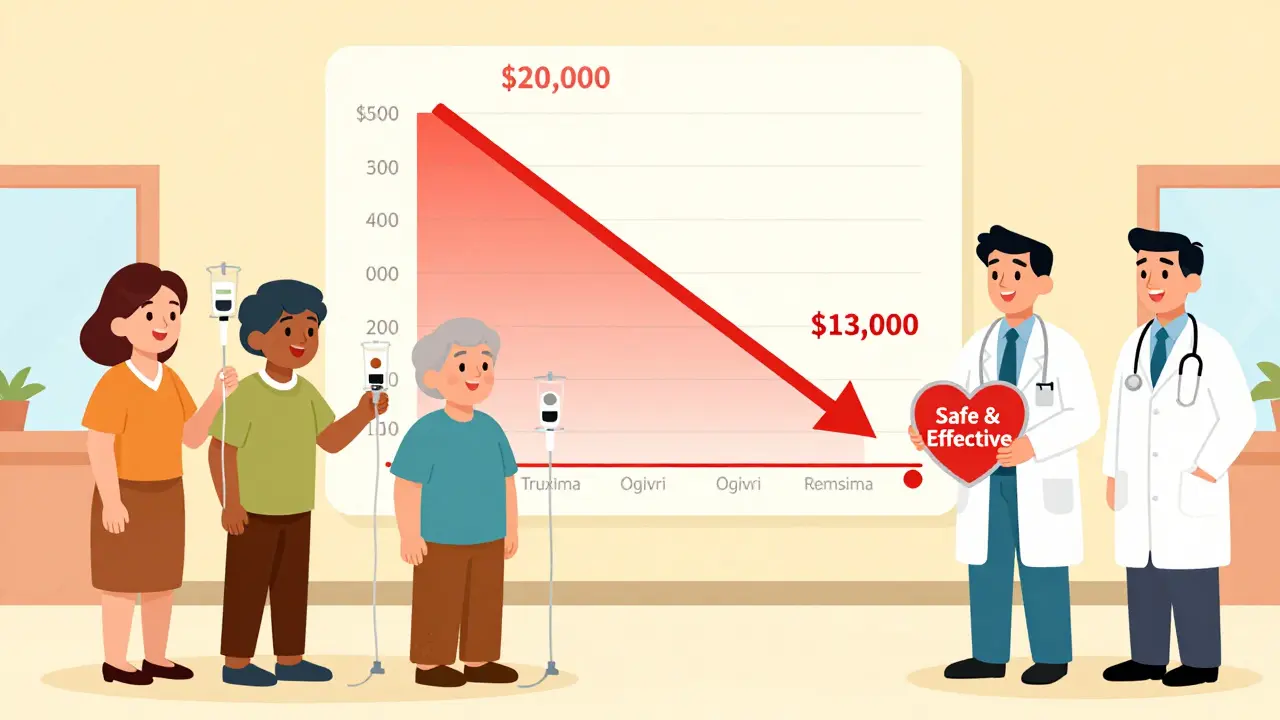
Are They Safe? What About Side Effects?
Some doctors worry about immune reactions. After all, these are proteins - your body might see them as foreign. But data from over 1.2 million patient-years of exposure shows biosimilar immune reactions occur at the same rate as the originals: about 0.001%. That’s less than one in a thousand patients. There was one case in 2011 where a patient had a severe allergic reaction to cetuximab because of a sugar structure called alpha-1,3-galactose. That led to stricter testing for glycosylation patterns. Today, every biosimilar must be tested for over 120 structural features, including sugar chains, protein folding, and charge variants. The FDA now recommends 127 specific analytical tests just to confirm similarity.Challenges Still Facing Biosimilars
Despite the science, adoption isn’t automatic. Three big barriers remain:- Patent battles: Companies that make the original drugs file lawsuits to delay biosimilar entry. On average, each monoclonal antibody biosimilar faces 14.7 patent challenges before it can launch.
- Provider hesitation: A 2022 survey found only 58% of oncologists felt very confident prescribing biosimilars. Many still believe they’re “less effective,” even though every study says otherwise.
- Insurance rules: Pharmacy benefit managers sometimes block biosimilars unless the patient tries the brand first - even though guidelines say biosimilars are equally safe.
Education is slowly changing this. Professional groups like ASCO and the Biosimilars Council now offer free training for doctors and pharmacists. Hospitals are adding biosimilar protocols to their cancer care pathways.

The Future: What’s Coming Next?
The pipeline is packed. As of late 2023, 37 monoclonal antibody biosimilars are under FDA review. The big targets are:- Pembrolizumab (Keytruda) biosimilars: Six are in late-stage trials. Keytruda alone brought in over $20 billion in 2023. Biosimilars could cut that price in half.
- Bispecific antibodies and antibody-drug conjugates: These are next-gen drugs that attach toxins directly to cancer cells. The EMA plans to release new guidelines for these complex biosimilars in early 2024.
By 2027, IQVIA predicts monoclonal antibody biosimilars will make up 35% of all biologic prescriptions in the U.S. - up from 18% in 2022. Cancer treatments will drive 62% of that growth.
Final Thought: It’s Not About Cheaper - It’s About Access
Monoclonal antibody biosimilars aren’t just cost-cutting tools. They’re equity tools. In Australia, where I live, the PBS (Pharmaceutical Benefits Scheme) now lists several biosimilars for rheumatoid arthritis and colon cancer. Patients who once waited months for approval now get treatment within weeks. The science is solid. The data is clear. The savings are real. The only thing holding back more patients is outdated thinking - not the medicine itself.Are monoclonal antibody biosimilars the same as generic drugs?
No. Generic drugs are chemically identical to their brand-name counterparts and are made from simple molecules. Monoclonal antibody biosimilars are complex proteins made by living cells. They’re highly similar to the original, but not identical - and regulators require far more testing to prove they work the same way in the body.
Can biosimilars be switched with the original drug safely?
Yes - if they’re labeled as “interchangeable.” The FDA has approved Remsima (infliximab) as interchangeable, meaning pharmacists can substitute it for the brand without a doctor’s new prescription. For non-interchangeable biosimilars, a doctor must specifically prescribe the biosimilar. Studies show switching doesn’t increase side effects or reduce effectiveness.
Do biosimilars have the same side effects as the original?
Yes. Clinical trials and real-world data show that side effects - including infusion reactions, infections, and immune responses - occur at the same rates as the reference product. The EMA and FDA both confirm there’s no increased risk of adverse events with biosimilars.
Why are biosimilars cheaper if they’re so complex to make?
They’re not cheaper to develop - in fact, they cost $100 million to $250 million to bring to market, compared to $2 million for a generic. But they’re cheaper to sell because manufacturers don’t have to repeat expensive clinical trials. They use data from the original drug’s trials, cutting development time and cost. That savings gets passed to patients and payers.
Are biosimilars approved in other countries?
Yes. The EMA approved the first monoclonal antibody biosimilar (infliximab) in 2013. Since then, over 50 biosimilars have been approved in the EU, with 35% being monoclonal antibodies. Canada, Japan, Australia, and many other countries have similar approval systems. The U.S. and EU standards are the most rigorous globally.
Laia Freeman
28 January / 2026OMG I JUST READ THIS AND I’M CRYING?? Like, I have a cousin on trastuzumab and we thought we’d never afford it… then we got Herzuma and it was like a miracle. THANK YOU to whoever made this post!!! <3