Tentative Approval: What It Means for Generic Drugs and Your Health
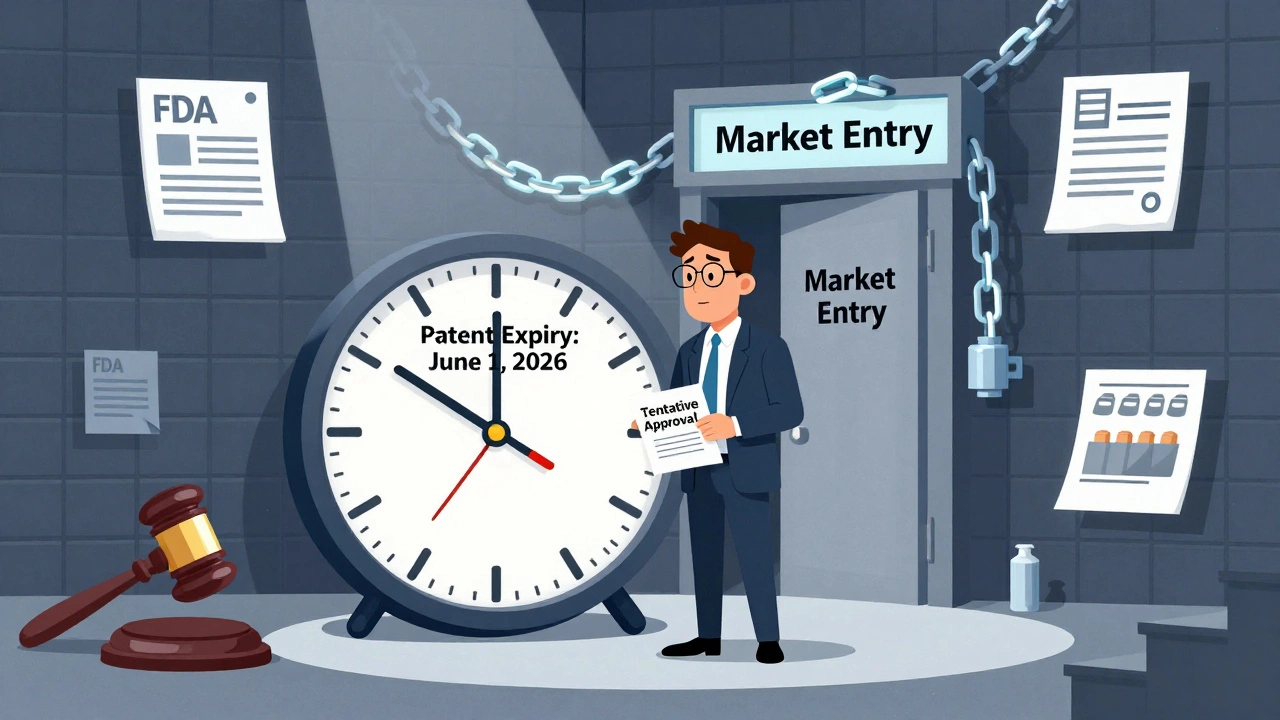
When a generic drug gets tentative approval, a status given by the FDA when a generic drug meets all safety and efficacy standards but can't yet be sold due to patent or exclusivity blocks. Also known as partial approval, it means the drug is ready to go—just waiting for the legal green light. This isn’t a delay in quality. It’s a delay in timing. The FDA has already checked the manufacturing, the active ingredients, and how the drug behaves in your body. Everything checks out. But someone else still holds the patent, so you can’t buy it yet.
Tentative approval isn’t rare. In fact, it’s common for popular generic drugs like metformin, lisinopril, or amoxicillin. The FDA grants it to companies that submit complete applications early, so they can hit the market the moment the patent expires. This system saves months, sometimes years, of waiting. And that means faster access to cheaper meds for you. When multiple companies get tentative approval for the same drug, prices drop fast—sometimes by over 70% once they launch. That’s the power of competition, and it’s built right into the process.
But here’s what most people don’t realize: tentative approval doesn’t mean the drug is approved for all uses. A company might get tentative approval for a 500mg tablet but not for the 250mg version. Or they might get approval for treating high blood pressure but not for heart failure. That’s why you can’t assume a generic with tentative approval will work the same way as the brand—until it’s fully approved. Always check the label and talk to your pharmacist.
And it’s not just about price. Tentative approval is a signal that a drug is on the way. If you’re on a medication that’s about to go generic, you might hear your doctor or pharmacist mention it. That’s good news. It means you’ll soon have a cheaper option that works the same. But it also means you should keep taking your current drug until the new one is actually on the shelf. Switching too early can cause problems—especially with drugs that have a narrow therapeutic index, like warfarin or levothyroxine. You need the real thing, not the soon-to-be version.
The FDA’s tentative approval system keeps the pipeline moving. It rewards companies that do their homework early. It protects patients from unsafe or untested generics. And it ensures that when a drug finally hits the market, it’s not just cheap—it’s reliable. That’s why you see so many posts here about generic drugs, medication journals, and how to track your response after switching. All of it connects back to this moment: when a drug is ready, but not yet available. That’s tentative approval. And it’s one of the quietest, most important parts of how you get affordable medicine.
Below, you’ll find real stories and guides from people who’ve been there—switching to generics, catching errors, saving money, and understanding exactly what happens when a drug moves from tentative to full approval. These aren’t theory pieces. They’re practical tools you can use right now.
Tentative approval from the FDA lets generic drug makers prepare for market entry before patents expire, but litigation and timing mistakes can cause costly delays. Learn how the Hatch-Waxman Act shapes the race to bring affordable drugs to patients.
View Details