High Eye Pressure Management
When dealing with high eye pressure management, the process of keeping intraocular pressure within safe limits to protect vision. Also known as IOP control, it blends medication, monitoring and lifestyle tweaks to lower the risk of damage.
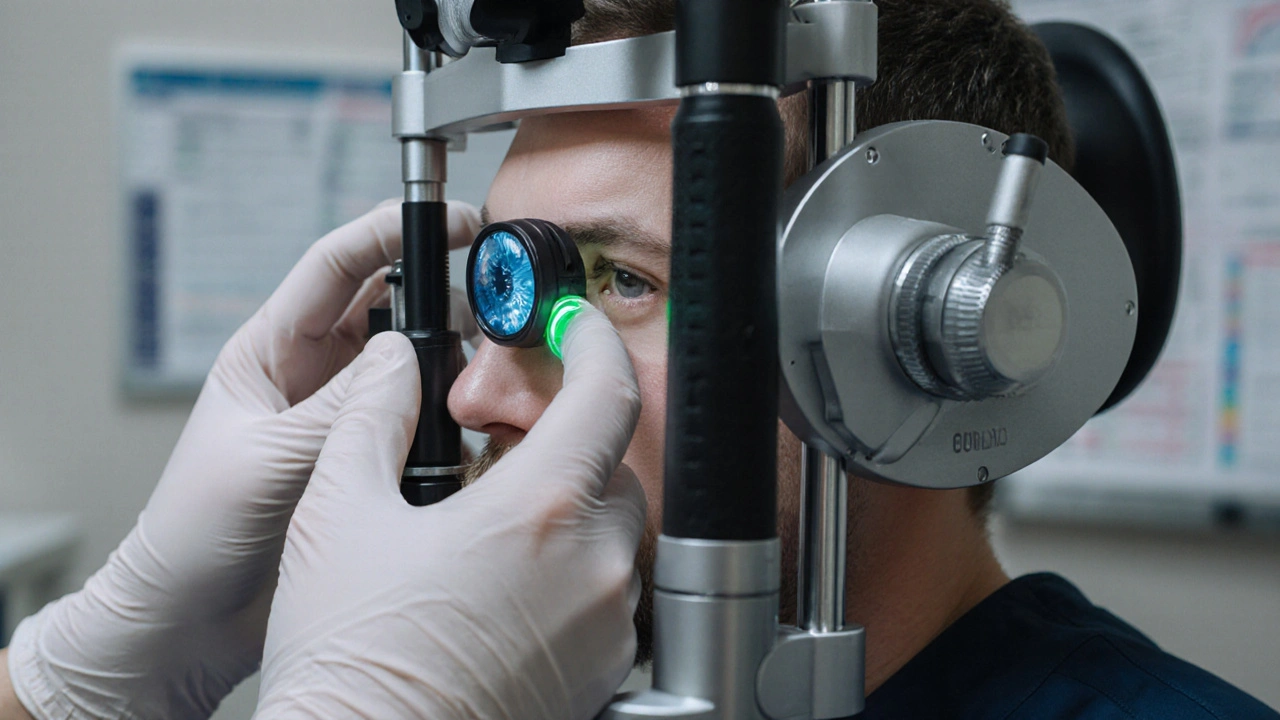
One of the biggest players in this field is glaucoma, a group of eye diseases where sustained high pressure can damage the optic nerve. Glaucoma typically shows up as either open‑angle or angle‑closure, each requiring slightly different treatment plans. Linked closely is intraocular pressure, the actual pressure measurement measured in millimeters of mercury; normal ranges sit between 10–21 mm Hg, and anything above signals the need for action. Managing this pressure often starts with eye drops, pharmaceutical formulations that either reduce fluid production or improve outflow. Common classes include prostaglandin analogues, beta‑blockers, carbonic anhydrase inhibitors and alpha‑agonists, each with its own side‑effect profile.
Key Components of Effective Management
First, regular monitoring builds the data set you need to adjust treatment. Many eye doctors recommend checking IOP at least twice a year for low‑risk patients and more often for those with progressive disease. The second component is medication adherence; skipping doses can cause pressure spikes that accelerate optic nerve loss. Third, procedural options such as laser trabeculoplasty or minimally invasive glaucoma surgery become valuable when drops alone don’t hit target pressures. Finally, lifestyle factors like caffeine intake, exercise, and sleep posture can subtly influence pressure—though they’re not a substitute for medical therapy, they serve as useful adjuncts.
From an attribute standpoint, high eye pressure management has three core attributes: measurement, intervention, and prevention. The measurement attribute revolves around tonometry readings, which can be taken with Goldmann applanation or newer non‑contact devices. Intervention covers pharmacologic agents (eye drops) and procedural techniques (laser, surgery). Prevention includes patient education, regular follow‑ups, and lifestyle tweaks that lower pressure spikes. For example, a study published in 2023 showed that a 30‑minute brisk walk lowered average IOP by 1 mm Hg in a group of glaucoma patients, highlighting the preventive potential of exercise.
Semantic connections flow naturally: high eye pressure management requires consistent IOP monitoring; IOP monitoring informs medication adjustments; medication adjustments reduce the risk of glaucoma progression. Likewise, laser therapy offers an alternative when drops fail to achieve target pressure, and lifestyle changes support the overall therapeutic plan.
If you’re new to the topic, start by asking your eye doctor for a baseline pressure reading and a clear target range. Next, discuss which eye drop class fits your health profile—some patients can’t tolerate prostaglandin analogues due to iris pigmentation changes, while others may prefer a once‑daily regimen for convenience. If you’re already on drops, track your pressure at home if you have a portable tonometer, and note any side effects like burning or blurred vision. Those clues help your doctor decide whether to add a second medication, consider laser trabeculoplasty, or explore surgical options.
For seasoned patients, the focus shifts to fine‑tuning. Small pressure fluctuations—often missed on a quarterly visit—can be caught with self‑monitoring and logged in an app. Over time, patterns emerge that reveal how caffeine, stress, or nighttime positioning affect your numbers. Adjusting coffee consumption, managing stress through meditation, or sleeping with the head slightly elevated can shave off a fraction of a millimeter of mercury, which matters when you’re already close to the target.
In the realm of drug choices, prostaglandin analogues like latanoprost or bimatoprost usually provide the strongest pressure drop (about 25‑30%). If a patient experiences eyelash growth or darkening of the iris, a beta‑blocker such as timolol may be swapped in, though it’s not suitable for people with asthma or certain heart conditions. Carbonic anhydrase inhibitors—both oral and topical—offer an additional 10‑15% reduction but can cause systemic side effects like tingling or taste changes. Combining agents from different classes often yields additive pressure lowering without significantly increasing side effects.
Laser therapy, especially selective laser trabeculoplasty (SLT), is gaining traction because it can reduce pressure by 20‑30% with a single office visit and has a low complication rate. It works by targeting pigmented cells in the trabecular meshwork, improving fluid outflow. For those with angle‑closure glaucoma, laser peripheral iridotomy creates a new pathway for fluid, preventing acute pressure spikes. Both procedures are considered safe adjuncts when medication alone isn’t enough.
Finally, remember that high eye pressure management is a lifelong partnership. Your eye care professional, the medications, and your daily habits all play distinct yet interconnected roles. By staying informed, adhering to treatment, and making small lifestyle tweaks, you give your eyes the best chance to stay healthy.
Below you’ll find a curated list of articles covering everything from medication buying guides to specific eye health topics. Whether you’re looking for the latest on safe online pharmacy purchases or need a deep dive into eye‑related conditions, the collection below offers practical insights you can act on right away.
Explore how laser treatments like SLT and ALT lower eye pressure, who qualifies, what to expect during the procedure, and how they compare to eye‑drop therapies.
View Details