Generic Drug Patent Litigation: How Lawsuits Shape Your Medication Costs
When you buy a generic drug, a chemically identical version of a brand-name medicine that costs far less. Also known as generic medication, it becomes available only after the original patent expires—unless a company blocks it with a lawsuit. This is where generic drug patent litigation, legal battles between brand-name drugmakers and companies trying to sell cheaper versions. Also known as pharmaceutical patent disputes, these cases decide who gets to sell the drug first and at what price. These aren’t just courtroom dramas—they directly affect whether you pay $5 or $50 for your blood pressure pill.
Brand-name companies often file lawsuits to delay generic entry, even when their patents are weak. They might claim a new formulation, a different dosage, or an unproven method of use—anything to stretch protection. These tactics, called "evergreening," keep prices high. But when generic manufacturers challenge these claims successfully, prices can drop over 70% within months, as seen with drugs like Lipitor and Plavix. The FDA tracks these cases, and when multiple generic makers enter the market, competition drives prices even lower. This system works well—until it doesn’t. Sometimes, a single generic company gets exclusive rights for six months, creating a temporary monopoly. Other times, lawsuits drag on for years, leaving patients stuck with expensive brand-name drugs.
These legal fights involve more than just companies. Patients, pharmacies, insurers, and even the government all feel the impact. A delay in generic approval means higher Medicaid and Medicare costs. It means people skip doses because they can’t afford their meds. It means pharmacists spend hours explaining why a cheaper option isn’t available yet. And it means you might be taking a brand-name drug not because it’s better—but because the courts haven’t ruled yet.
What you’ll find in the posts below isn’t just about lawsuits. It’s about the real-world ripple effects: how patent delays affect drug availability, why some generics cost more than others, how competition lowers prices, and what you can do when your medication suddenly becomes unaffordable. From how multiple manufacturers slash costs to how direct-to-consumer pharmacies bypass middlemen, these stories show the hidden mechanics behind your prescription bottle. This isn’t abstract law—it’s your health, your budget, and your right to affordable care.
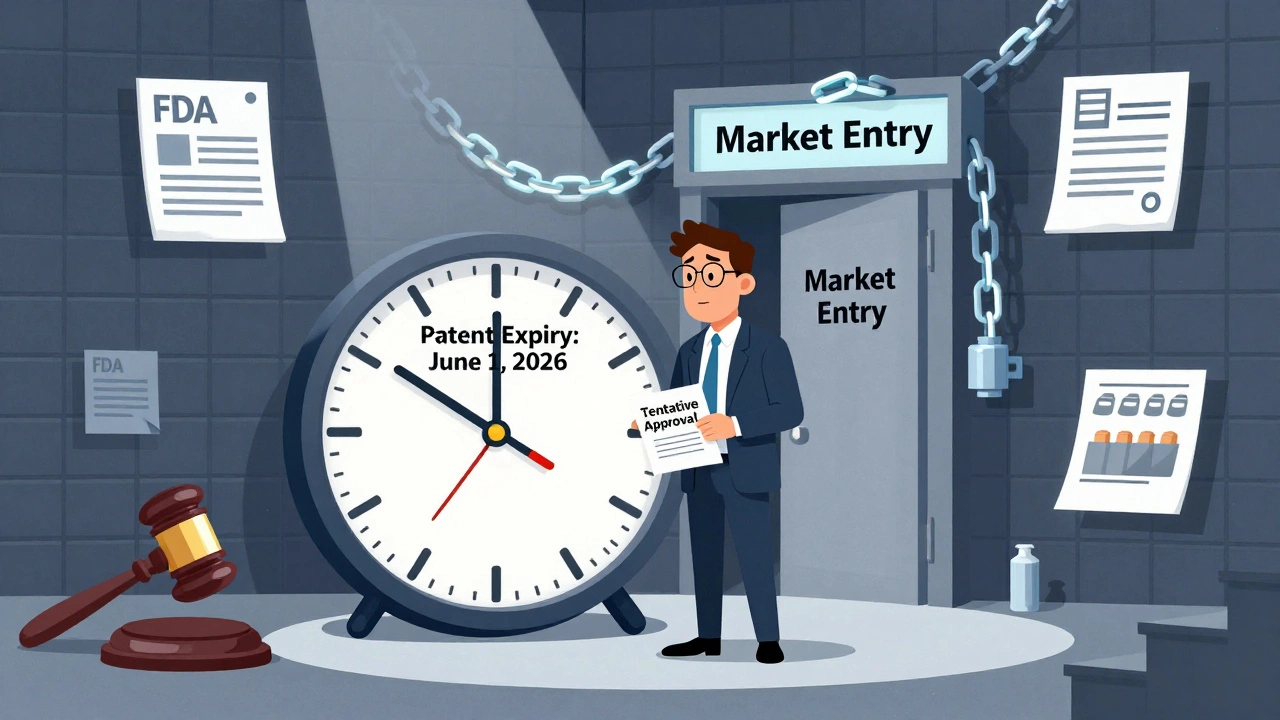
Tentative approval from the FDA lets generic drug makers prepare for market entry before patents expire, but litigation and timing mistakes can cause costly delays. Learn how the Hatch-Waxman Act shapes the race to bring affordable drugs to patients.
View Details