Tag: bioequivalence standards
29 Jan 2026
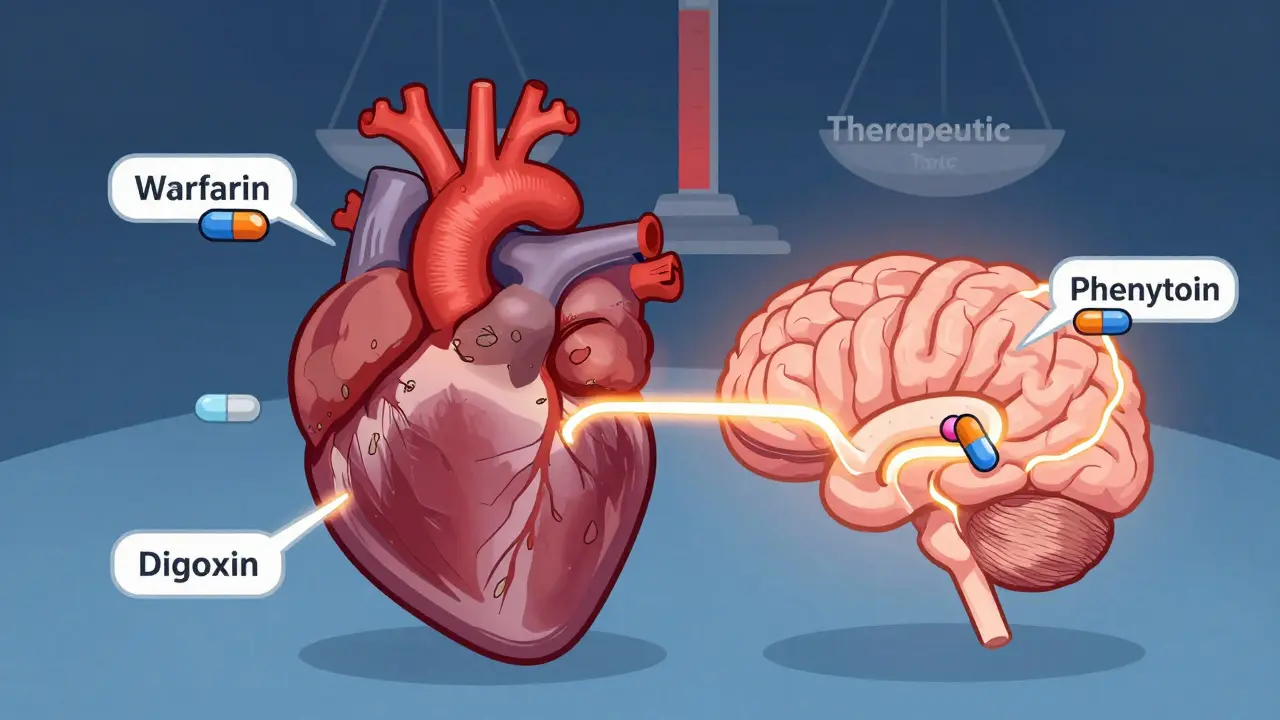
The FDA enforces stricter bioequivalence rules for NTI drugs like warfarin, digoxin, and phenytoin, requiring tighter potency limits and variability controls to ensure patient safety. Learn how these standards differ from regular generics.
View Details