FDA ANDA: What It Means for Generic Drugs and Your Wallet
When you pick up a generic pill at the pharmacy, chances are it got there because of an FDA ANDA, a streamlined application process the U.S. Food and Drug Administration uses to approve generic versions of brand-name drugs. Also known as an Abbreviated New Drug Application, it’s how the FDA confirms that a generic drug works the same way as the original—without forcing manufacturers to repeat every single clinical trial. This isn’t just paperwork. It’s the reason you can fill a 30-day supply of metformin for $4 instead of $300.
The FDA ANDA doesn’t skip safety. It just skips what’s already proven. The generic maker must show their drug has the same active ingredient, strength, dosage form, and route of administration as the brand-name version. They also prove it’s absorbed the same way in your body and works just as well. That’s why a generic amoxicillin from Cenmox or any other brand treats strep throat just as effectively as the original. The FDA doesn’t approve generics based on cost—they approve them based on science.
And here’s the kicker: the system works because of competition. When multiple companies file an ANDA for the same drug—like pravastatin or lamotrigine—the market floods with options. Prices drop fast. One study showed that when four or more manufacturers enter the market, prices fall by over 70%. That’s not magic. That’s the ANDA process doing its job. It’s why you can find coupons for generic drugs but not for brand-name biologics—those still need full new drug applications, not abbreviated ones.
But the ANDA doesn’t just affect price. It affects safety too. If a generic drug causes a side effect, or if it interacts badly with another medication like 5-HTP or MAO inhibitors, that report goes straight to the FDA. Patients and doctors can file those reports through MedWatch, and the FDA uses that data to update labels, issue warnings, or even pull a drug off the market. That’s why your medication journal matters—it’s part of the feedback loop that keeps the whole system honest.
Some people worry that generics are "inferior." But if that were true, you’d see more cases of Stevens-Johnson Syndrome or toxic epidermal necrolysis tied to generics. You’d see more liver damage from opioids because of inconsistent absorption. You wouldn’t see doctors confidently prescribing generic versions of complex drugs like carbidopa-levodopa-entacapone for Parkinson’s pain. The truth? The FDA ANDA process is one of the most rigorously enforced systems in healthcare.
What you’ll find below are real stories and practical guides about how this system touches your life. From how to report a bad reaction to how multiple manufacturers drive down prices, from why you should carry pills in original containers while traveling to how switching generics can affect your health—every post here connects back to one thing: the FDA ANDA isn’t just a form. It’s the quiet engine behind every affordable, safe generic drug you take.
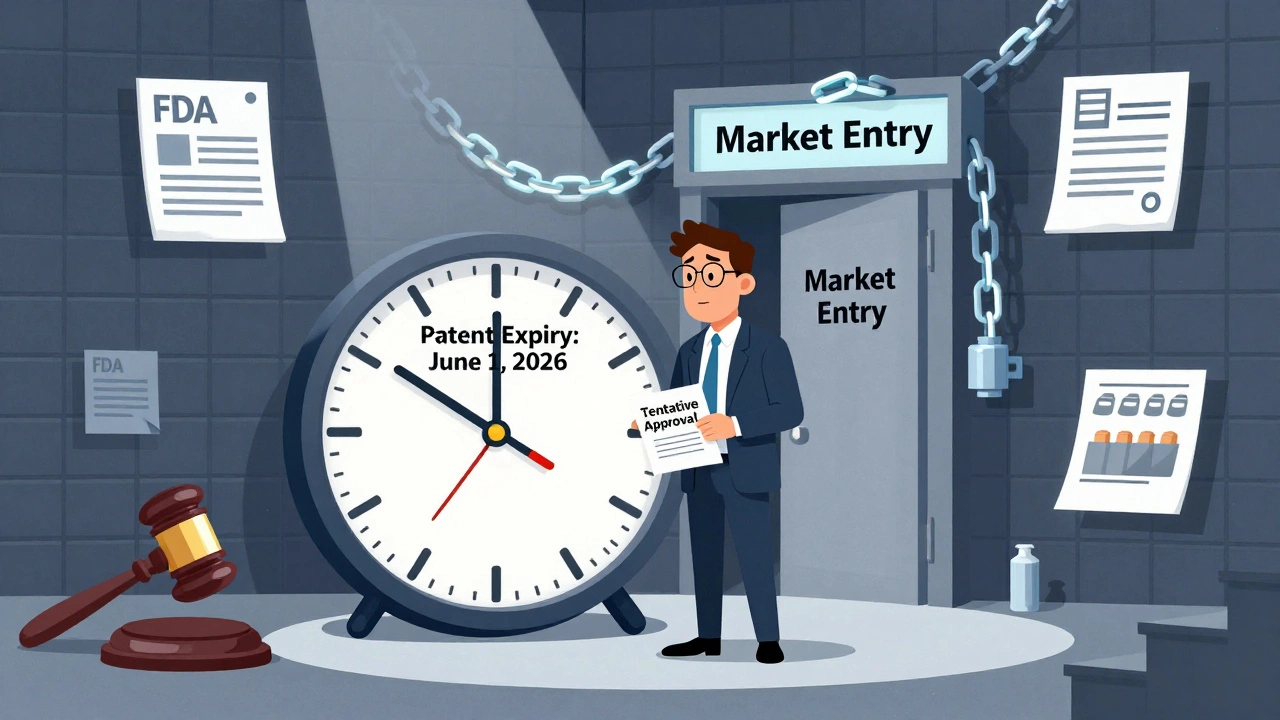
Tentative approval from the FDA lets generic drug makers prepare for market entry before patents expire, but litigation and timing mistakes can cause costly delays. Learn how the Hatch-Waxman Act shapes the race to bring affordable drugs to patients.
View Details